Human Mesothelial Cells
Mesothelial cells play pivotal roles in ovarian cancer metastasis, peritoneal dialysis, surgical adhesions, inflammatory response, and metabolic disease. These specialized epithelial cells form a single cell layer with a critical barrier function and provide a frictionless surface for organs and tissue to move without damage. We isolate peritoneal mesothelial cells from omental tissue biopsies with minimal propagation.
Mesothelial cells are available either cryopreserved or in culture. Ready to use medium is also available for use with our mesothelial cells.
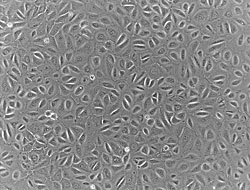
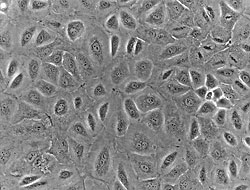
Human mesothelial cells in culture
Ordering Information:
Human Cryopreserved Mesothelial Cells
| Item# | Item Desc | U/M | Price |
|---|---|---|---|
| MSO-1 | Mesothelial Cell Growth Medium | 500ml | $199.00 |
| MSB-1 | Mesothelial Cell Basal Medium | 500ml | $162.00 |
| MCM-100 | Mesothelial Cell Cryopreservation Medium | 100ml | $269.00 |
Human Adult Mesothelial Care Manual
Contact Us For More Information.
Human Mesothelial Cells Publications
Arachidonic acid drives adaptive responses to chemotherapy-induced stress in malignant mesothelioma
Mario Cioce, Claudia Canino, Harvey Pass, Giovanni Blandino, Sabrina Strano & Vito Michele Fazio DOI:https://doi.org/10.1186/s13046-021-02118-y
Endothelin-1 drives invadopodia and interaction with mesothelial cells through ILK
Ilenia Masi, Valentina Caprara, Francesca Spadaro, Lidia Chellini, Rosanna Sestito, Andrea Zancla, Alberto Rainer, Anna Bagnato, Laura Rosanòhttps://doi.org/10.1016/j.celrep.2021.108800
The Cell Line-Dependent Diversity in Initial Morphological Dynamics of Pancreatic Cancer Cell Peritoneal Metastasis Visualized by an Artificial Human Peritoneal
Tadashi Odagiri, Yoshiya Asano, Takuji Kagiya, Michiya Matsusaki, Mitsuru Akashi, Hiroshi Shimoda, Kenichi Hakamadahttps://doi.org/10.1016/j.jss.2020.12.046
Injured tissues favor cancer cell implantation via fibrin deposits on scar zones
Iman Al dybiat, Shahsoltan Mirshah, Meriem Belalou, Djedjiga Abdelhamid, Shahid Shah, Matti Ullah, Jeannette Soria, Marc Pocard, Massoud Mirshahihttps://doi.org/10.1016/j.neo.2020.09.006ITLN1 modulates invasive potential and metabolic reprogramming of ovarian cancer cells in omental microenvironment
Chi-Lam Au-Yeung, Tsz-Lun Yeung, Abhinav Achreja, Hongyun Zhao, Kay-Pong Yip, Suet-Ying Kwan, Michaela Onstad, Jianting Sheng, Ying Zhu, Dodge L. Baluya, Ngai-Na Co, Angela Rynne-Vidal, Rosemarie Schmandt, Matthew L. Anderson, Karen H. Lu, Stephen T. C. Wong, Deepak Nagrath & Samuel C. MokDOI DOIhttps://doi.org/10.1038/s41467-020-17383-2
PDK1 promotes ovarian cancer metastasis by modulating tumor-mesothelial adhesion, invasion, and angiogenesis via α5β1 integrin and JNK/IL-8 signaling
Michelle K. Y. Siu, Yu-xin Jiang, Jing-jing Wang, Thomas H. Y. Leung, Siew Fei Ngu, Annie N. Y. Cheung, Hextan Y. S. Ngan & Karen K. L. ChanGenomic mapping identifies multiple therapeutic pathways in malignant mesothelioma
Anca Nastase, Amit Manda, Shir Kiong Lu, Hima Anbunathan , Deborah Morris-Rosendahl, Yu Zhi Zhang, Xiao-Ming Sun, Spyridon Gennatas, Robert C Rintoul, Matthew Edwards, Alex Bowman, Tatyana Chernova, Tim Benepal, Eric Lim, Anthony Newman Taylor, Andrew G Nicholson, Sanjay Popat, Anne E Willis, Marion MacFarlane, Mark Lathrop, Anne M Bowcock, Miriam F Moffatt, William OCM Cooksondoi: http://dx.doi.org/10.1101/2020.01.23.2001852
Host Wnt5a Potentiates Microenvironmental Regulation of Ovarian Cancer Metastasis
Marwa Asem, Allison M. Young, Carlysa Oyama, Alejandro G Claure De La Zerda, Yueying Liu, Jing Yang, Tyvette S. Hilliard, Jeffery Johnson, Elizabeth I. Harper, Ian Guldner, Siyuan Zhang, Toni M Page-Mayberry, William J. Kaliney and M. Sharon StackDOI: 10.1158/0008-5472.CAN-19-1601
Activation of General Control Nonderepressible-2 Kinase Ameliorates Glucotoxicity in Human Peritoneal Mesothelial Cells, Preserves Their Integrity, and Prevents Mesothelial to Mesenchymal Transition
Theodoros Eleftheriadis, Georgios Pissas, Georgia Antoniadi, Evdokia Nikolaou, Spyridon Golfinopoulos, Vassilios Liakopoulos and Ioannis StefanidisBiomolecules 2019, 9, 832; doi:10.3390/biom9120832
Fibrin Deposit on the Peritoneal Surface Serves as a Niche for Cancer Expansion in Carcinomatosis Patients
Shah Shahid, Aldybiat Iman, Ullah Matti, Kaci Rachid, Alassaf Assaf, Clarisse Eveno,Pocard Marc, Mirshahi Massoudhttps://doi.org/10.1016/j.neo.2019.08.006
Inhibitory effect of carbonyl reductase 1 against peritoneal progression of ovarian cancer: evaluation by ex vivo 3D-human peritoneal model
Hiroe Oikiri, Yoshiya Asano, Michiya Matsusaki, Mitsuru Akashi, Hiroshi Shimoda, Yoshihito YokoyamaDOI: https://doi.org/10.1007/s11033-019-04788-6
High Expression of HLA-G in Ovarian Carcinomatosis: The Role of Interleukin-1β
Matti Ullah, Dallel Azazzen, Rachid Kaci, Nadia Benabbou, Eric Pujade, Lauraine Marc Pocard, Massoud Mirshahihttps://doi.org/10.1016/j.neo.2019.01.001
Preclinical investigation of folate receptor-targeted nanoparticles for photodynamic therapy of malignant pleural mesothelioma
Tatsuya Kato, Cheng S. Jin, Daiyoon Lee, Hideki Ujiie, Kosuke Fujino, Hsin-Pei Hu, Hironobu Wada, Licun Wu, Juan Chen, Rober A. Weersink, Hiromi Kanno, Yutaka Hatanaka, Kanako C. Hatanaka, Kichizo Kaga, Yoshiro Matsui, Yoshihiro Matsuno, Marc De Perrot, Brian C. Wilson, Gang Zheng, Kazuhiro Yasufukuhttps://doi.org/10.3892/ijo.2018.4555
Long-Fiber Carbon Nanotubes Replicate Asbestos-Induced Mesothelioma with Disruption of the Tumor Suppressor Gene Cdkn2a (Ink4a/Arf)
Tatyana Chernova, Fiona A. Murphy, Sara Galavotti, Xiao-Ming Sun, Ian R. Powley, Stefano Grosso, Anja Schinwald, Joaquin Zacarias-Cabeza, Kate M. Dudek, David Dinsdalehttps://doi.org/10.1016/j.cub.2017.09.007
Construction of artificial human peritoneal tissue by cell-accumulation technique and its application for visualizing morphological dynamics of cancer peritoneal ...
Yoshiya Asano, Tadashi Odagiri, Hiroe Oikiri, Michiya Matsusaki, HiroshiShimodahttps://doi.org/10.1016/j.bbrc.2017.10.050
Joint analysis of left ventricular expression and circulating plasma levels of Omentin after myocardial ischemia
Louis A. Saddic, Sarah M. Nicoloro, Olga T. Gupta, Michael P. Czech, Joshua Gorham, Stanton K. Shernan, Christine E. Seidman, Jon G. Seidman, Sary F. Aranki, Simon C. Body, Timothy P. Fitzgibbons†and Jochen D. MuehlschlegelCardiovascular Diabetology201716:87
DOI: 10.1186/s12933-017-0567-x
Measuring non-polyaminated lipocalin-2 for cardiometabolic risk assessment
Kangmin Yang, Han-Bing Deng, Andy W.C. Man, Erfei Song, Jialiang Zhang, Cuiting Luo, Bernard M.Y. Cheung, Kwok-Yung Yuen, Pia Søndergaard Jensen, Akhmadjon Irmukhamedov, Atlanta G.I.M. Elie, Paul M. Vanhoutte, Aimin Xu, Jo G.R. De Mey, Yu WangDOI: 10.1002/ehf2.12183
Analysis of early mesothelial cell responses to Staphylococcus epidermidis isolated from patients with peritoneal dialysis-associated peritonitis
Amanda L. McGuire , Kieran T. Mulroney, Christine F. Carson, Ramesh Ram, Grant Morahan, Aron Chakerahttps://doi.org/10.1371/journal.pone.0178151
Obesity Contributes to Ovarian Cancer Metastatic Success through Increased Lipogenesis, Enhanced Vascularity, and Decreased Infiltration of M1 Macrophages
Yueying Liu1,2, Matthew N. Metzinger2, Kyle A. Lewellen1,2, Stephanie N. Cripps3, Kyle D. Carey2, Elizabeth I. Harper4, Zonggao Shi1,2, Laura Tarwater2, Annie Grisoli2,5, Eric Lee2, Ania Slusarz6,7, Jing Yang1,2, Elizabeth A. Loughran1,2, Kaitlyn Conley1,2, Jeff J. Johnson1,2, Yuliya Klymenko2,5, Lana Bruney2,7, Zhong Liang1,2, Norman J. Dovichi1,2, Bentley Cheatham8, W. Matthew Leevy2,5, and M. Sharon Stack1,2,*doi: 10.1158/0008-5472.CAN-15-0706